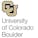Course 2 of Statistical Thermodynamics presents an introduction to quantum mechanics at a level appropriate for those with mechanical or aerospace engineering backgrounds. Using a postulatory approach that describes the steps to follow, the Schrodinger wave equation is derived and simple solutions obtained that illustrate atomic and molecular structural behavior. More realistic behavior is also explored along with modern quantum chemistry numerical solution methods for solving the wave equation.



Quantum Mechanics
This course is part of Statistical Thermodynamics Specialization

Instructor: John W. Daily
Sponsored by MAHE Manipal
22,445 already enrolled
(716 reviews)
What you'll learn
Describe the relationship between the Schrödinger wave equation and atomic/molecular structural behavior
Demonstrate an understanding of modern quantum chemistry numerical solution methods for solving the wave equation
Analyze the role of quantum mechanics in explaining atomic and molecular structural behavior
Details to know

Add to your LinkedIn profile
1 assignment
See how employees at top companies are mastering in-demand skills

Build your subject-matter expertise
- Learn new concepts from industry experts
- Gain a foundational understanding of a subject or tool
- Develop job-relevant skills with hands-on projects
- Earn a shareable career certificate


Earn a career certificate
Add this credential to your LinkedIn profile, resume, or CV
Share it on social media and in your performance review

There are 3 modules in this course
Module 1presents an introduction to quantum mechanics at a level appropriate for those with mechanical or aerospace engineering backgrounds. Using a postulatory approach that describes the steps to follow, the Schrodinger wave equation is derived and it is showen that the time dependence can be separated and a stationary wave equation results.
What's included
4 videos4 readings1 assignment1 discussion prompt
In module 2 we solve the stationary wave equation for several simple systems. These include the particle in a box, the rigid rotator, the harmonic oscillator, and the hydrogenic atom. These simple solutions form the basisi for discussing real atomic and molecular behavior in the next module.
What's included
4 videos4 readings1 discussion prompt
In Module 3 we explore the more realistic behavior of atoms and molecules. We also introduce and discuss numerical methods for solving the wave equation.
What's included
6 videos6 readings2 discussion prompts
Instructor

Offered by
Why people choose Coursera for their career




Learner reviews
716 reviews
- 5 stars
69.97%
- 4 stars
20.67%
- 3 stars
4.88%
- 2 stars
1.25%
- 1 star
3.21%
Showing 3 of 716
Reviewed on Jul 15, 2020
it's a good course for a basic and conceptual understanding of quantum mechanics.
Reviewed on Sep 19, 2020
it's nice organised and guided us through, it could be clearer in some illustration and explanation.
Reviewed on May 24, 2020
This Course on Quantum Mechanics is well organized and easy to understand. Course has been excellently delivered by Instructor. I recommend this course to physicist.
Recommended if you're interested in Physical Science and Engineering
University of Colorado Boulder

Arizona State University
Ludwig-Maximilians-Universität München (LMU)
Duke University

Open new doors with Coursera Plus
Unlimited access to 10,000+ world-class courses, hands-on projects, and job-ready certificate programs - all included in your subscription
Advance your career with an online degree
Earn a degree from world-class universities - 100% online
Join over 3,400 global companies that choose Coursera for Business
Upskill your employees to excel in the digital economy